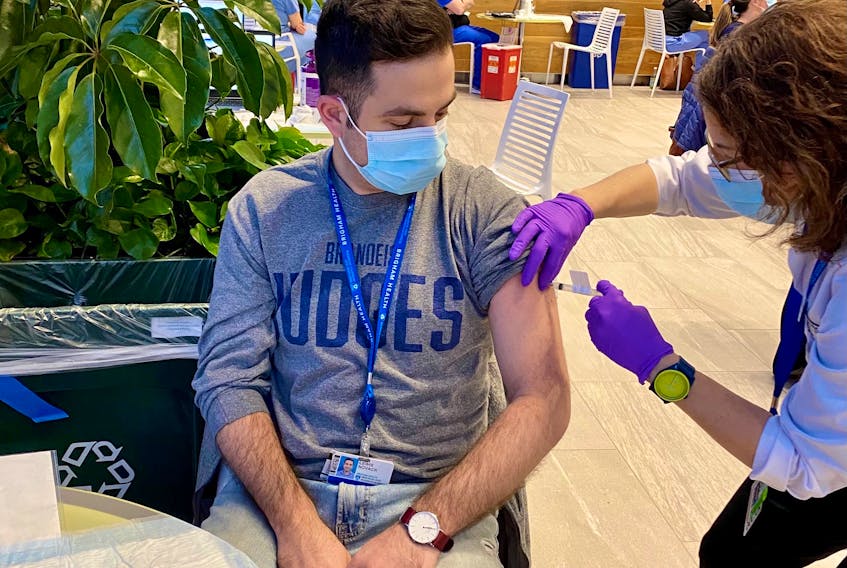
As a clinical epidemiologist, Lewis Novack was happy to roll up his sleeve recently and get his Moderna COVID-19 shot.
But he understands the uncertainty around these drugs that have been fast-tracked through clinical trials in order to combat the viral pandemic that’s raging around the world.
“When we hear about the speed of developing (a vaccine) and injecting it into people in less than a year it’s definitely worrisome because we don’t fully know the effects of this long-term, although we have a pretty good idea of what to expect,” said Novack, a Halifax native who works at the Harvard University-affiliated Brigham and Women’s Hospital in Boston.
“It’s a scary thing, it’s a new concept, it’s a new vaccine. … We hope to protect everyone but we’re still injecting a substance in those people so I understand people’s hesitancy.”
Novack worked on clinical trials of the Moderna vaccine, which is now in use in many countries including the United States and Canada. The epidemiological work at Harvard focused on diversity and inclusion in recruiting volunteers for vaccine trials.
“This virus has affected populations disproportionately,” said Novack, who after graduating from Armbrae Academy in Halifax earned an honours degree from Carleton University in anthropology and a masters degree in global health policy management from Brandeis University in Boston.
“We see communities that have been disadvantaged, people who need to go to work because they don’t have the financial stability to stay home and play it safe. These are the people we’re seeing in ICU, people being admitted to hospital, so we really want to enrol those people and give trust back to them.”
As part of his health policy studies, he worked closely with the Gates Foundation and started to specialize in infectious and non-infectious diseases that affect different populations such as HIV in African countries. That work led him to the Harvard School of Public Health where he studied neglected tropical infectious diseases.
Possible 'game-changer'
He now manages vaccine trials at Brigham hospital and his current project involves a vaccine produced by Janssen, the pharmaceutical arm of Johnson and Johnson, which he said could be a “game-changer” in the fight against COVID-19.
The Pfizer and Moderna vaccines require a “cold-chain” storage and delivery system that uses ultra-low temperature freezers. People also need two doses for maximum effectiveness.
The Janssen drug doesn’t require ultra-low temperature storage and the Harvard trials use a single-dose regimen (although Janssen is also studying a double-dose approach at other sites).
“The cold-chain aspects are very, very problematic,” Novack said, noting that doctors' offices and rural hospitals in many parts of the United States don’t have the storage facilities to keep vaccines at -80 C.
“They don’t have the set-up for this. Once you thaw the vaccine, it’s only available for a couple of hours, six hours at the most.”
Researchers are "very hopeful” the Janssen drug can achieve the high effectiveness rates found in the clinical trials of the Moderna and Pfizer vaccines.
But the long-term, on-the-ground effectiveness of any COVID-19 vaccine won’t be known for a long time.
“We don’t know how long these antibodies which the vaccine produces, how long that stays in our system,” Novack said. “Is this going to be a six-month thing, which I hope it’s not, or is it going to be a 10-year thing, or maybe it’s a lifetime thing. These are questions that we still need answers to and this is why it’s important that participants stay in the trials and we do what we can to give them the vaccine and track them over time to understand these results.”